Assistant Professor
Degrees and Appointments
- Assistant Professor, 2022, University of Arizona
- Ph.D., 2010 -2014, ETH Zürich, Switzerland
- B.S and M.Sc., 2010, ETH Zürich, Switzerland
Awards and Honors
- 2020 - 2025 K99/R00 NIGMS Pathway to Independence Award
- 2015 - 2017 Swiss National Science Foundation. Early Postdoc Mobility Fellowship
Alzheimer’s disease (AD) is a fatal neurodegenerative disease affecting 5.5 million Americans. Despite many decades of research there is still no known cure. AD is a protein misfolding disease, where the Alzheimer’s protein, Ab, aggregates from a random coil entity into fibrils, which are highly organized aggregates containing a cross-b sheet structure. However, the nature of the toxic species in Alzheimer’s disease remains unknown. Nature has developed mechanisms to prevent disease-associated protein aggregation, e.g. by the introduction of heat shock proteins (Hsp’s), which are overexpressed when cells undergo stress. Hsp60 is the only essential chaperone in bacteria, yeast, and mammals. It is known that Hsp60 is cytoprotective against many stressors in cells and is proposed to be directly protective against AD. However, nothing is known about the mechanism of how this is achieved.
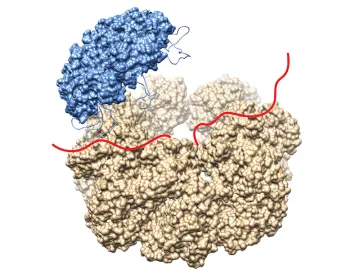
We will be using a variety of biochemical assays to investigate how Hsp60 interacts with the Alzheimer’s peptide with a focus on solution-state NMR and cryo-electron microscopy. These studies will provide insights into fundamental questions about the mechanism chaperones use to efficiently fold proteins into their functional forms. Further the results will unveil details about how Hsp60 inhibits the aggregation of the Alzheimer’s peptide and prevents neurodegenerative disease and may open up novel therapeutic strategies against Alzheimer’s disease. Read More on Hegetschweiler (née Wälti) Group Webpage
Marielle Aulikki Wälti, Bertram Canagarajah, Charles D. Schwieters, G. Marius Clore, Visualization of sparsely-populated oligomeric assembly intermediates of human mitochondrial Hsp60 by cryo-electron microscopy. 2021, J Mol Biol., 433(24):167322
Marielle Aulikki Wälti, Joseph Steiner, Fanjie Meng, Hoi Sung Chung, John M. Louis, Rodolfo Ghirlando, Vitali Tugarinov, Avindra Nath, G. Marius Clore. Probing the Mechanism of Inhibition of Amyloid b(1-42) Induced Neurotoxicity by the Chaperonin GroEL, 2018, Proc Natl Acad Sci U S A. pii: 201817477
Marielle Aulikki Wälti*, Francesco Ravotti*, Hiromi Arai, Charles Glabe, Joseph Wall, Anja Böckmann, Peter Güntert, Beat H. Meier, Roland Riek. Atomic resolution structure of a disease-relevant Amyloid-β(1-42) amyloid fibril. 2016, Proc Natl Acad Sci U S A; 113(34):E4976-84
Marielle Aulikki Wälti, Samuel Kotler, G. Marius Clore. Probing the interaction of huntingtin exon-1 polypeptides with the chaperonin nanomachine GroEL. 2021, Chembiochem, 22(11):1985-1991
Marielle Aulikki Wälti, Thomas Schmidt, Dylan T. Murray, Huaibin Wang, Jenny E. Hinshaw, G. Marius Clore. Chaperonin GroEL Accelerates Protofibril Formation and Decorates Fibrils of the Het-s Prion Protein, 2017, Proc Natl Acad Sci U S A ;114(34):9104-9109
Marielle Aulikki Wälti, Dvaid Libich, G. Marius Clore. Extensive sampling of the cavity of the GroEL nanomachine by protein substrates probed by paramagnetic relaxation enhancement, 2018, J Phys Chem Lett. 9(12):3368-3371
Julien Orts, Marielle Aulikki Wälti, May Marsh, Laura Vera, Alvar D. Gossert, Peter Güntert, Roland Riek. NMR-Based Determination of the 3D Structure of the Ligand-Protein Interaction Site without Protein Resonance Assignment. 2016, J. Am. Chem. Soc. 138(13):4393-400
Marielle Aulikki Wälti, Roland Riek, Julien Orts. Fast NMR-Based Determination of the 3D Structure of the Binding Site of Protein-Ligand Complexes with Weak Affinity Binders. 2017, Angew Chem Int Ed Engl. 56(19):5208-5211
Marielle Aulikki Wälti, Julien Orts, The NMR2 method to determine rapidly a complex structure of the binding pocket at high accuracy, Review, 2018, Magnetochemistry, 4(1), (Selected for cover illustration)






