Professor
Degrees and Appointments
- B.S. 2001, University of Trieste, Italy
- Ph.D. 2007, University of Texas at Austin
- Postdoctoral Associate 2007-2010, Massachusetts Institute of Technology (MIT)
Fields of Study: Inorganic Chemistry, Organic Chemistry
Research Specialities: Bioinorganic, Chemical Biology, Spectroscopy/Molecular Structure, Synthesis/Synthetic Methods Development
Awards and Honors:
- University Distinguished Scholar Award, 2021
- Donna B. Cosulich Faculty Fellow, 2020
- Young Observer, International Union of Pure and Applied Chemistry (IUPAC), 2019
- College of Science Innovation in Teaching Award, 2017
- Excellence in Campus Outreach for STEM Diversity Award, 2016
- NSF CAREER Award, 2015
- Young Investigator Award of the International Society for Zinc Biology, 2009
- Robert A. Welch Professional Development Fellowship, 2006
- Elio Russo Award, University of Trieste, 2002
Research
We have recently developed a class of antiproliferative chelators that scavenge intracellular iron ions upon reduction/activation by glutathione in cancer cells. This approach, which is aimed at the development of new molecular tools for the study and treatment of cancer, seeks to exploit an emerging vulnerability of malignant cells, namely their altered iron metabolism and increased demand for this essential metal ion. The disulfide bonds employed as redox switches in this pro-chelator design can also serve to attach tumor-targeting units and impart increased cancer selectivity. We have recently prepared glucose conjugates that target the overexpression of glucose transporters in colon cancer cells.
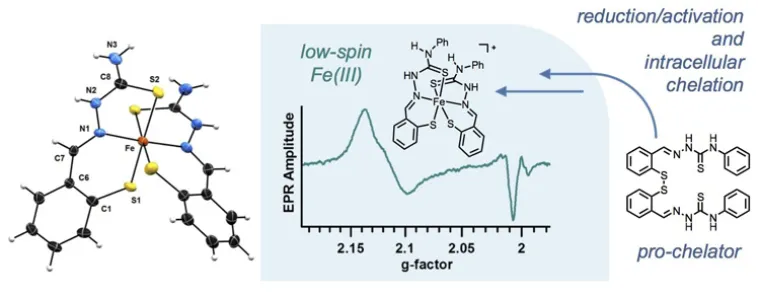
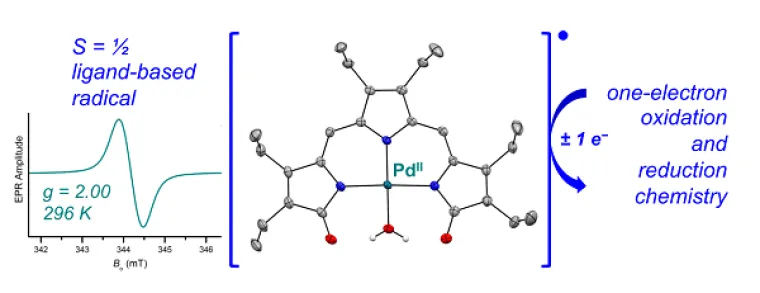
We are inspired by bioinorganic chemistry for the design of novel metal complexes featuring both metal-based and ligand-based redox chemistry. Our current work in this area is focused on naturally occurring pyrrole-based compounds (e.g., bacterial prodigiosins, biopyrrins from heme metabolism) and on their role as redox-active ligands in coordination chemistry and catalysis. For instance, we have demonstrated that the tripyrrindione class of heme metabolites can coordinate transition metals as dianionic radicals that are stable at room temperature. We are currently examining the reactivity of these metal complexes as well as their potential in redox catalysis and functional materials.
Because of the interdisciplinary nature of our queries, the design of research plans in the Tomat laboratory combines principles of synthetic and coordination chemistry with current knowledge of cell metabolism and molecular biology. We employ the tools of synthetic chemistry and a variety of spectroscopic techniques (e.g., NMR, EPR, UV-visible absorption, fluorescence emission) and electrochemical methods for the preparation and characterization of metal complexes both in vitro and in cultured mammalian cells.
Jung, M.; Mertens, C.; Tomat, E.; Brüne, B. “Iron as a central player and promising target in cancer progression” Int. J. Mol. Sci. 2019, 20, 273.
Gautam, R.; Petritis, S. J.; Tomat*, E. “Redox-switchable cyan fluorescence of a BODIPY analog inspired by propentdyopent pigments” Eur. J. Inorg. Chem. 2019, 68-72.
Gautam, R.; Petritis, S. J.; Astashkin, A. V.; Tomat*, E. “Paramagnetism and Fluorescence of Zinc(II) Tripyrrindione: A Luminescent Radical Based on a Redox-active Biopyrrin” Inorg. Chem. 2018, 57, 15240-15246 (cover feature).
Akam, E. A.; Utterback, R.; Marcero, J. R.; Dailey, H. R.; Tomat*, E. “Disulfide-masked iron prochelators: Effects on cell death, proliferation, and hemoglobin production” J. Inorg. Biochem. 2018, 180, 186–193.
Gautam, R.; Astashkin, A. V.; Chang, T. M.; Shearer, J.; Tomat*, E. “Interactions of metal-based and ligand-based electronic spins in neutral tripyrrindione π dimers” Inorg. Chem. 2017, 56, 6755–6762.
Akam, E. A., Tomat*, E. “Targeting iron in colon cancer via glycoconjugation of thiosemicarbazone prochelators” Bioconjugate Chem. 2016, 27, 1807–1812.
Mertens, C.; Akam, E. A.; Rehwald, C.; Brüne, B.; Tomat*, E.; Jung*, M. “Intracellular iron chelation modulates the macrophage iron phenotype with consequences on tumor progression” PLoS ONE 2016, 11, e0166164.
Gautam, R.; Chang, T. M.; Astashkin, A. V.; Lincoln, K. M.; Tomat*, E. “Propentdyopent: The scaffold of a heme metabolite as an electron reservoir in transition metal complexes” Chem. Commun. 2016, 52, 6585–6588.
Tomat*, E. “Coordination chemistry of linear tripyrroles: Promises and perils” Comments Inorg. Chem. 2016, 36, 327–342.
Gautam, R.; Loughrey, J. J.; Astashkin, A. V.; Shearer, J.; Tomat*, E. “Tripyrrindione as a redox-active ligand: Palladium(II) coordination in three redox states” Angew. Chem. Int. Ed. 2015, 54, 14894–14897.
Gautam, R.; Akam, E. A.; Astashkin, A. V.; Loughrey, J. J.; Tomat*, E. “Sirtuin inhibitor sirtinol is an intracellular iron chelator” Chem. Commun. 2015, 51, 5104–5107.
Akam, E. A.; Chang, T. M.; Astachkine, A. V.; Tomat*, E. "Intracellular reduction/activation of a disulfide switch in thiosemicarbazone iron chelators" Metallomics 2014, 6, 1905-1912.






