Professor
Degrees and Appointments
- B.S. 1986, Yale University
- Ph.D. 1992, California Institute of Technology
- Postdoctoral 1992-1994, The Scripps Research Institute
- Postdoctoral 1994, California Institute of Technology
Field of Study: Organic Chemistry
Awards and Honors
- Camille Dreyfus Teacher Scholar Award, 1999-2004
- Research Corporation Cottrell Scholar, 1997-2002
- NSF CAREER Award, 1997-2002
Research Specialties: Energy Science, Materials and Polymer Chemistry, Synthesis/Synthetic Methods Development
Research
Our research program involves the use of organic synthesis for the design, development, and application of new concepts in macromolecular, supramolecular, and materials chemistry. Our research efforts span a number of areas in the chemical sciences and include studies of (a) materials for solar energy conversion, (b) macromolecular systems that undergo structural changes in response to visible light and other stimuli, and (c) the influence of dendritic components of nanoscopic systems on phonic and electronic properties of materials.
We have developed several new classes of dendritic materials containing photochromic subunits. As nature uses light energy to alter function in photoresponsive systems such as photosynthesis, vision, phototropism, and phototaxis, we use light energy to drive gross topological or constitutional changes in fundamentally new dendritic architectures with precisely placed photoresponsive subunits. In short, we can drive dendrimer properties with light stimuli. We have developed two entirely new classes of photoresponsive dendritic macromolecules: (1) Photochromic Dendrimers, and (2) Photolabile Dendrimers. We anticipate that switchable and degradable dendrimers of this type will have application in small molecule transport systems based on their ability to reversibly encapsulate guest molecules. We are continuing to develop these materials as potential transport hosts and photoresponsive supramolecular assemblies.
Our interest in labile macromolecular structures has led to our development of the process of dendrimer disassembly, whereby a triggering stimulus initiates an electronic cascade cleavage of dendritic structures into individual dendrimer subunits or larger dendrimer fragments. This chemistry introduces a new paradigm for the use of dendritic structures based on (1) the nature of dendrimers as covalent assemblages of active species, and using the chemistry of disassembly to release these species into a system; and (2) the role of dendritic components of a system in influencing solubility, energy harvesting, or insulating capabilities, etc., and using the chemistry of disassembly to reverse those contributions to a system. This is a powerful construct, in that dendrimers and dendritic structures can be made up of a wide variety of subunits, compatibilized with many different environments, and incorporated into countless systems. We anticipate that dendritic materials with disassembly capabilities will (a) be useful for traditional polymer degradation technologies, and (b) have potential applications in nanotechnology, biomedicine, sensors, etc.
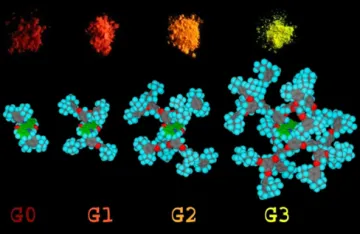
Reduced aggregation of a quinacridone core in dendrimers of increasing generation enhances solid state luminescence efficiency [Chem. Commun. 2005 444]
“Non-symmetric Pyrene-fused Pyrazaacenes via Green Oxidation of 2,7-di-tert-butylpyrene,” El-Assaad, T.H.; McGrath, D.V. J. Org. Chem. 2024, 89, 1989-1992.
“Enhancing perovskite solar cell performance through dynamic hydrogen-mediated polarization of nitrogen and sulfur in phthalocyanine.” Qu, G.; Qiao, Y.; Zeng, J.; Cai, S.; Chen, Q.; Wang, D.; Khan, D.; Huang, L.; Xu, B.; Chen, J.; El-Assaad, T.; Wang, Y.-G.; McGrath, D. V.; Xu, Z.-X., Nano Energy, 2023, 118, 108974.
“Dioxiranes: A Half-Century Journey,” El-Assaad, T.H.; Zhu, J.; Sebastian, A.; McGrath, D.; Neogi, I.; Parida, K. Org. Chem. Front. 2022, 9, 5675-5725.
“Beyond Simple Structure-Function Relationships: The Interplay of Geometry, Electronic Structure and Molecule/Electrode Coupling in Single Molecule Junctions,” Bamberger, N.; Dyer, D.; Parida, K.; El-Assaad, T.; Pursell, D.; McGrath, D.; Smeu, M.; Monti, O. J. Phys. Chem. C 2022, 126, 6653-66661.
“Phenalenyls as tunable excellent molecular conductors and switchable spin filters,” Smeu, M.; McGrath, D.V.; Monti, O.L.A. PhysChemChemPhys. 2021, 23, 24106-24110.
“Grid-Based Correlation Analysis to Identify Rare Quantum Transport Behaviors," Bamberger, N.D.; Dyer, D.; Parida, K.N.; McGrath, D.V.; Monti, O.L.A. J. Phys. Chem. C 2021, 125, 18297–18307.
"Correlated Energy-Level Alignment Effects Determine Substituent-Tuned Single-Molecule Conductance," Ivie, J.A.; Bamberger, N.D.; Parida, K.N.; Shepard, S.; Dyer, D.M.; Saraiva-Souza, A.; Himmelhuber, R.; McGrath,* D.V.; Smeu,* M.; Monti,* O.L.A. ACS Adv. Mater. Interfaces 2021, 13, 4267-4277.
"Dual Defect-passivation Using Phthalocyanine for Enhanced Efficiency and Stability of Perovskite Solar Cells," Hu,Q; Rezaee, E.; Xu, W.; Ramachandran, R.; Chen, Q.; Xu, H.; El-Assaad, T.; McGrath,† D.V.; Xu,† Z.-X. Small 2020, 17, 2005216.
"Unsupervised Segmentation-Based Machine Learning as an Advanced Analysis Tool for Single Molecule Break Junction Data," Nathan D. Bamberger, Jeffrey A. Ivie, Keshaba N. Parida, Dominic V. McGrath, and Oliver L. A. Monti† J. Phys. Chem. C 2020, 124, 18302−18315.
"Sterically Driven Metal-Free Oxidation of 2,7-Di-tert-butylpyrene,” El-Assaad, T.H.; Parida, K.N.; Cesario, M.F.; McGrath,† D.V. Green Chem. 2020, 22, 5966-5971.
"A Molecular Design Strategy in Developing Titanyl Phthalocyanines as Dopant-Free Hole Transporting Materials for Perovskite Solar Cells: Peripheral or Non-Peripheral Substituents?," Hu, Q.; Rezaee, E.; Li, M.; Chen, Q.; Cao, Y.; Mayukh, M.; McGrath,† D. V.; Xu,† Z.-X., ACS Appl. Mater. Interfaces 2019, 11, 36535-36543.
"Zinc Phthalocyanine-Phosphonic Acid Monolayers on ITO: Influence of Molecular Orientation, Aggregation, and Tunneling Distance on Charge-Transfer Kinetics," Oquendo, L.E.; Ehamparam, R.; Armstrong, N.R.; Saavedra, S.S.; McGrath,† D.V. J. Phys. Chem. C. 2019, 123, 6970-6980.
"Axially Bound Ruthenium Phthalocyanine Monolayers on Indium Tin Oxide: Structure, Energetics, and Charge Transfer Properties," Ehamparam, R.; Oquendo, L.E.; Liao, M.W.; Brynnel, A.K.; Ou, K.-L.; Armstrong, N.R.; McGrath,† D.V.; Saavedra, S.S. ACS Appl. Mater. Interfaces 2017, 9, 29213-29223.
"2-Photon Characterization of Optical Proteolytic Beacons for Imaging Changes in MMP Activity in a Mouse Model of Aneurysm," Haskett, D.G; Maestas, D.; Howerton, S.; Smith, T.; Ardilia, C.; Doetschman, T.; Utzinger, U.; Dominic McGrath,D.; McIntyre, J.O.; Vande Geest, J.P. Microscopy and Microanalysis 2016, 22, 349-360.
"Solution Processed Titanyl Phthalocyanine Derivatives as Donors in Solar Cells: Photoconversion to 1000 nm," Mayukh, M.; Macech, M.; Placencia, D.; Cao, Y.; Armstrong, N.R.; McGrath, D.V. ACS Appl. Mater. Interfaces 2015, 7, 23912-23919.
“Modification of alkyne-functionalized asymmetric phthalocyanines by CuI-catalyzed azide-alkyne cycloaddition,” Chen, X.; Lu, C.-W.; Huang, Y.; McGrath, D.V. Tetrahedron 2015, 71, 9154-9160.
"Asymmetric ZnPc-TEG photosensitizers: The effect of Pc substitution on phototoxicity," Muli, D.K.; Rajaputra, P.; You, Y.; McGrath, D.V. Tetrahedron Lett. 2015, 56, 6236-6239.
“Influence of Molecular Orientation on Charge Transfer Processes at Phthalocyanine/Metal Oxide Interfaces and Relationship to Organic Photovoltaic Performance,” Lin, H.-C.; MacDonald, G.A.; Shi, Y.; Polaske, N.W.; McGrath, D.V.; Marder, S.R.; Armstrong, N.R.; Ratcliff, E.L.; Saavedra, S.S. J. Phys. Chem. C 2015, 119, 10304-10313.
"Dendritic Near-IR Absorbing Zinc Phthalocyanines for Antimicrobial Photodynamic Therapy," Muli, D.K.; Carpenter, B.; Ghiladi, R.; McGrath, D.V. Tetrahedron Lett. 2015, 56, 3541-3545.
"Asymmetric ZnPc-Rhodamine B conjugates for mitochondrial targeted photodynamic therapy," Muli, D.K.; Rajaputra, P.; You, Y.; McGrath, D.V. Bioorg. Med. Chem. Lett. 2014, 36, 4496-4500.
"Electron Transfer Processes in Zinc Phthalocyanine-Phosphonic Acid Monolayers on ITO: Characterization of Orientation and Charge Transfer Kinetics By Waveguide Spectroelectrochemistry," Lin, H.-C.; Polaske, N.W.; Oquendo, L.E.; Gliboff, M.; Knesting, K.M.; Nordlund, D.; Ginger, D.S.; Ratcliff, E.L.; Beam, B.M.; Armstrong, N.R.; McGrath, D.V.; Saavedra, S.S. J. Phys. Chem. Lett. 2012, 3, 1154-1158.
"Vanillin and o-vanillin oligomers as models for dendrimer disassembly," Kevwitch, R.M.; Shanahan, C.S.; McGrath, D.V. New J. Chem. 2012, 36, 492-505.
"Phosphonic Acid Functionalized Asymmetric Phthalocyanines: Synthesis, Modification of Indium Tin Oxide (ITO), and Charge Transfer," Polaske, N.W.; Lin, H.-C.; Tang, A.; Mayukh, M.; Oquendo, L.E.; Green, J.T.; Ratcliff, E.R.; Armstrong, N.R.; Saavedra, S.S.; McGrath, D.V. Langmuir 2011, 25, 14900-14909.
"Thermally Reversible Dendronized AB Step-Polymers via "Click" Chemistry," Polaske, N.W.; McGrath, D.V.; McElhanon, J.R. Macromolecules 2011, 44, 3203-3210.
"Peripheral Substitution of a Near-IR Absorbing Soluble Phthalocyanine Using 'Click' Chemistry," Mayukh, M.; Lu, C.-W.; Hernandez, E.; McGrath, D.V. Chem. Eur. J. 2011, 17, 8472-8478.
"Solvent-Free Synthesis of Soluble, Near-IR Absorbing Titanyl Phthalocyanine Derivatives," Mayukh, M.; Sema, C.M.; Roberts, J.M.; McGrath, D.V. J. Org. Chem. 2010, 75, 7893-7896.
"Convergent Synthesis of Geometrically Disassembling Dendrimers using Cu(I)-Catalyzed C-O Bond Formation," Polaske, N.W.; Szalai, M.L.; Shanahan, C.S.; McGrath, D.V. Org. Lett. 2010, 12, 4944-4947.
"Polymeric Endoaortic Paving (PEAP): Thermomechanical and Degradation Properties of Polycaprolactone/Polyurethane Blends for Cardiovascular Applications," Ashton, J.H.; Mertz, J.A.; Harper, J.L.; Slepian, M.J.; Mills, J.L.; McGrath, D.V.; Vande Geest, J.P. Acta Biomaterialia 2010, 7, 287-294.
"Improved Iterative Synthesis of Linearly Disassembling Dendrons," Ortiz, A.; Shanahan, C.S.; Sisk, D.T.; Perera, S.C.; Rao, P.; McGrath, D.V. J. Org. Chem. 2010, 75, 6154-6162.
"Frustration of Condensed Phase Aggregation of Naphthalocyanine by Dendritic Site-Isolation," Chen, X.; Fernando, N.; McGrath, D.V. Macromolecules 2010, 43, 5512-5514.






